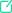题目信息
Given a positive number N, when N is rounded by a certain method (for convenience, call it Method Y), the result is 10n if and only if n is an integer and 5 × 10n − 1 ≤ N < 5 × 10n. In a certain gas sample, there are, when rounded by Method Y, 1021 molecules of H2 and also 1021 molecules of O2. When rounded by Method Y, what is the combined number of H2 and O2 molecules in the gas sample?
A:Statement (1) ALONE is sufficient, but statement (2) alone is not sufficient.
B:Statement (2) ALONE is sufficient, but statement (1) alone is not sufficient.
C:BOTH statements TOGETHER are sufficient, but NEITHER statement ALONE is sufficient.
D:EACH statement ALONE is sufficient.
E:Statements (1) and (2) TOGETHER are NOT sufficient.
参考答案及共享解析

共享解析来源为网络权威资源、GMAT高分考生等; 如有疑问,欢迎在评论区提问与讨论
本题耗时:
已选答案:
正确答案:
C:BOTH statements TOGETHER are sufficient, but NEITHER statement ALONE is sufficient.
Arithmetic Rounding
Let H be the number of H2 molecules and let O be the number of O2 molecules. We are given that 0.5 × 1021 ≤ H < 5 × 1021 and 0.5 × 1021 ≤ O < 5 × 1021. When rounded by Method Y, what is the value of H + O ?
If H = 2 × 1021 and O = 2 × 1021, then each of H and O equals 1021 when rounded by Method Y, each of H and O is less than 3 × 1021, and H + O = 4 × 1021, which equals 1021 when rounded by Method Y. However, if H = 2.6 × 1021 and O = 2.6 × 1021, then each of H and O equals 1021 when rounded by Method Y, each of H and O is less than 3 × 1021, and H + O = 5.2 × 1021, which equals 1022 when rounded by Method Y; NOT sufficient. If H = 2 × 1021 and O = 0.8 × 1021, then each of H and O equals 1021 when rounded by Method Y, H > 2 × O, and H + O = 2.8 × 1021, which equals 1021 when rounded by Method Y. However, if H = 4.5 × 1021 and O = 2 × 1021, then each of H and O equals 1021 when rounded by Method Y, H > 2 × O, and H + O = 6.5 × 1021, which equals 1022 when rounded by Method Y; NOT sufficient.
Given (1) and (2), since O ≥ 0.5 × 1021 (given information), it follows from statement (2) that H > 1 × 1021. Also, since H < 3 × 1021 (statement (1)), it follows from statement (2) that O < 1.5 × 1021. Thus, 1 × 1021 < H < 3 × 1021 and 0.5 × 1021 ≤ O < 1.5 × 1021, and hence 1.5 × 1021 < H + O < 4.5 × 1021. Therefore, the value of H + O equals 1021 when rounded by Method Y.
The correct answer is C;both statements together are sufficient.
Let H be the number of H2 molecules and let O be the number of O2 molecules. We are given that 0.5 × 1021 ≤ H < 5 × 1021 and 0.5 × 1021 ≤ O < 5 × 1021. When rounded by Method Y, what is the value of H + O ?
If H = 2 × 1021 and O = 2 × 1021, then each of H and O equals 1021 when rounded by Method Y, each of H and O is less than 3 × 1021, and H + O = 4 × 1021, which equals 1021 when rounded by Method Y. However, if H = 2.6 × 1021 and O = 2.6 × 1021, then each of H and O equals 1021 when rounded by Method Y, each of H and O is less than 3 × 1021, and H + O = 5.2 × 1021, which equals 1022 when rounded by Method Y; NOT sufficient. If H = 2 × 1021 and O = 0.8 × 1021, then each of H and O equals 1021 when rounded by Method Y, H > 2 × O, and H + O = 2.8 × 1021, which equals 1021 when rounded by Method Y. However, if H = 4.5 × 1021 and O = 2 × 1021, then each of H and O equals 1021 when rounded by Method Y, H > 2 × O, and H + O = 6.5 × 1021, which equals 1022 when rounded by Method Y; NOT sufficient.
Given (1) and (2), since O ≥ 0.5 × 1021 (given information), it follows from statement (2) that H > 1 × 1021. Also, since H < 3 × 1021 (statement (1)), it follows from statement (2) that O < 1.5 × 1021. Thus, 1 × 1021 < H < 3 × 1021 and 0.5 × 1021 ≤ O < 1.5 × 1021, and hence 1.5 × 1021 < H + O < 4.5 × 1021. Therefore, the value of H + O equals 1021 when rounded by Method Y.
The correct answer is C;both statements together are sufficient.
 加入收藏
加入收藏
 在线答疑
在线答疑
题目来源